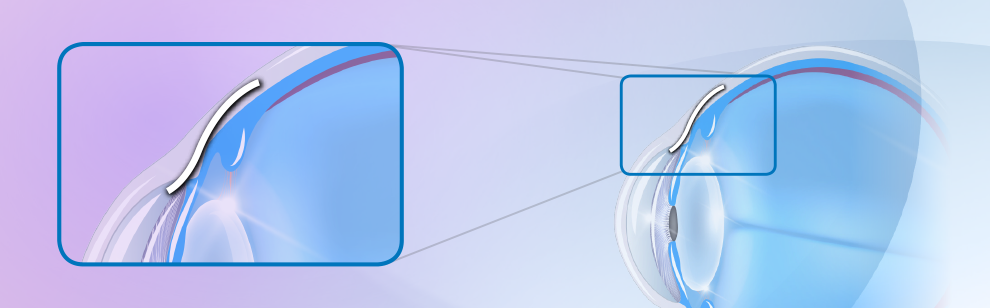
What is a Xen gel stent?
Xen gel stent is a FDA approved glaucoma procedure and is considered microinvasive glaucoma surgery (MIGS). The stent is made of a soft, non-inflammatory, gelatin like material. The 6mm stent is made to be placed permanently under the conjunctiva. Aqueous fluid from inside the eye is now able to flow through the stent and collect under the conjunctiva, creating a bleb or blister.
How is the surgery performed?
A small incision, similar to one made during cataract surgery, is made in the cornea (the clear front part of the eye). The device is then placed into the eye through the incision and guided to the angle of the eye. The stent is then injected through the trabecular meshwork (the drainage portion of the angle). The stent is advanced until one end is under the conjunctiva while the other end remains inside the eye. Fluid from inside the eye can now exit to the space under the conjunctiva. This creates a bleb where the fluid can collect and then be absorbed by the body. Anti-scarring medications, such as mitomycin, is applied to the conjunctiva to modulate healing and prevent scarring.
Am I candidate for a Xen gel stent?
The Xen gel stent is approved for patient with refractory glaucoma, including patient with open angle, pseudoexofoliation, or pigmentary glaucoma. It is not for patients with closed angle glaucoma, conjunctival scarring, or prior glaucoma tubes.
What are the risks of surgery?
As with all eye surgery, there is a risk of infection, bleeding, or decrease vision. There is a risk of scarring at the surgery site and failure. The surgery may not adequately control eye pressure and medications or additional procedures may be required. There is a small risk that the pressures is too low after surgery, which may need additional medications or procedures to resolve.

